Keith Parent is a Contributing Author to DIA’s eBook “Achieving Excellence in Regulatory Information Management.”
Court Square Group is proud to recognize Keith Parent as a contributing author to DIA’s newest eBook “Achieving Excellence in Regulatory Information Management.”
As experts in the industry, we are excited to be part of this publication that provides a comprehensive guide on best practices in regulatory information management (RIM) capabilities and functional interfaces.
Calling all members of the Regulatory Affairs Community! Dive into the latest release of the comprehensive DIA eBook that will provide insights into new topics that are shaping the industry.
Discover the evolution of the original RIM consensus white paper to its latest 3.0 publication, packed with best practices across the spectrum of RIM capabilities and functional interfaces.
Learn from regulatory affairs experts who have updated the RIM consensus white paper to include cutting-edge topics such as regulatory intelligence and automation.
Don’t miss out on this valuable resource that covers the latest trends and best practices in RIM capabilities. Grab your copy today!
Purchase the eBook today!
Table of Contents
- Defining the Scope of Regulatory Information Management (RIM)
- Development of a Vision and Outline for a RIM Framework
- Regulatory Intelligence
- Health Authority Interactions – Correspondence and Commitments
- Submission Planning and Tracking
- Submission Authoring, Publishing, Dispatch, and Archiving
- Labeling
- The Complex Labeling Process
- Advertising and Promotional Material
- Product Registration Management
- Change Control and Variation Management
- Cross-Functional Touchpoints
- Regulatory Analytics and Dashboards
- Data Quality and Data Governance
- Intelligent Automation in Regulatory
- Medical Devices
- Digitalization and Data Standards
- Health Authority Collaborations
- Systems Connectivity
- Real-World Data Usage in Regulatory Information Management
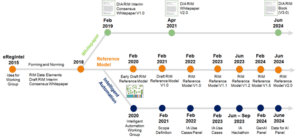
The purpose of the DIA Regulatory Affairs Community RIM Working Group was to define industry best practices and develop a common understanding of RIM. The Working Group embarked on three workstreams: a RIM Consensus Whitepaper, a RIM Reference Model, and Intelligent Automation. The following graphic shows the timeline of the three workstreams with respective milestones and deliverables:
Get Your Copy of the eBook Today!

About Court Square Group
Court Square Group (CSG) is the leading provider of an Audit Ready Compliant Cloud™ (ARCC) platform for Life Science companies. The ARCC cloud platform and out-of-the-box tools provide a validated and cost-effective way to manage all digital content (EDMS/documents, voice, data, and video) in a regulated and compliant environment. CSG’s cloud, collaboration and regulatory submission solutions reduce costs, complexity and risks associated with sharing, storing, and submitting information for regulatory requirements. With over 1,000+ submissions and twenty-five years’ experience and a 95% client retention rate, CSG has a proven track record as one of the most cost-efficient solutions in the life science market.


